Code 86328 was established as a child code to 86318 to report a qualitative or semiquantitative single-step method immunoassay for SARS-CoV-2 COVID-19 antibodyies. December 3 2020.
Pakistan Lab S Mess Up Leaves Sharjah Ticket Buyer From Mar Menafn Com
Punjab Forensic Science Auth Lab Jinnah Hospital PKLI Lahore General Hospital CAMB UVAS BSL-3 Lahore TB Program BSL-3 IPH Lahore Chughtai Lab Shaukat Khanum Memorial Hospital Rahila Research and Reference Lab Venus Diagnostics Hameed Lateef Hospital Agha Khan Citilab Research Centre Surgimed Lab Zeenat Lab Genome Lab Testzone Al Nasar Lab.
Chughtai lab coronavirus test report pdf. 39 Years 2582020 12200PM 2582020 24930PM. This will help us keep all of your information in one record. COVID 19 Laboratory Capacity National Institute of Health Islamabad 1 January 2021 S.
Laboratory testing is an integral part of this strategy. CDC requires every COVID-19 testing site to report every diagnostic and screening test performed to detect SARS-CoV-2 or to diagnose a possible case of COVID -19 eg molecular antigen antibody to the appropriate state or local public health department based on the individuals residence. This policy brief discusses the role of testing for COVID 19 as part of any plan to lift confinement restrictions and prepare for a possible new wave of viral infections.
Diagnostic Immunology Laboratory requisition pdf. COVID-19 caused by SARS-CoV-2. On average this form takes 40 minutes to.
All forms are printable and downloadable. Clinical Laboratory order form pdf COVID-19 patient testing requisition pdf Allergens IgE test requisition pdf Cancer and Blood Diseases Show. Human Infection with 2019 Novel Coronavirus Person Under Investigation PUI and Case Report Form.
133A Faisal Town Opposltc Allama Iqbal Mcdical College Lahore 042-35163747 03364820296 List of Test Zone Lab Branches For COVID-19 PCR Puniab. Tests that generate PDF reports. Medical staff members take information from a resident sitting in a car for a coronavirus test at a drive-through testing facility in Karachi.
The purpose of collating this information is to help maintain ongoing public health and safety. The following document contains a list of these tests. Final 18 Years 2432020 100600AM 2432020 11922PM MKPDP1035 Male DrVeena Bora DUMMY 2432020 12321PM Test Name Results Units Bio.
The death toll as a result of coronavirus is 1526 while 8365. If Chughtai Lab has perfomed a test for you in the past please locate your Patient Number and Case Number in the top right corner of your receipt. Medicalsurgical masks and respirators are commonly used as protection against respiratory and other infections.
Each country should assess its risk and rapidly implement the necessary measures at the. SARS COV-2 COVID-19 TOTAL ANTIBODIES IgGIgM SERUM Results to follow. CARES Act Section 18115 January 8 2021 Assuring a rapid and thorough public health response to the COVID-19 pandemic necessitates complete and comprehensive laboratory testing data including standardized test results relevant demographic details and additional information that can improve both the.
The citizen can view and download their COVID RATI test report in pdf format online through this portal. Mandatory Reporting of COVID-19 Laboratory Test Results. If you cant find your patient number and case number you can call us at.
However private laboratories are charging PKR 7900 for the tests. Public National Institute of Health - NIH PIMS POCT PCR Private IDC 4. Name of HospitalTesting Laboratory Location of HospitalTesting Laboratory I have been fully informed that as a requirement of travel with Qatar Airways Group QCSC Qatar Airways I must where requested presentsubmit valid COVID-19 PCR test results to Qatar Airways.
Advanced Laboratories Gulshan-e-Iqbal Block 15. According to the Express Tribune government hospitals are conducting coronavirus tests in Pakistan for free. IMPORTANT INSTRUCTIONS Test results released pertain to the specimen submittedAll test results are dependent on the quality of the sample received by.
Code 86769 was established to report an antibody test for SARS-CoV-2 Coronavirus disease COVID-19 using a multiple-step method. View RATI Test Report. Report Status Reported Received Collected P.
If you or someone you know is showing COVID-19 symptoms contact health officials immediately. Laboratories are subject to mandatory reporting to the Florida Department of Health FDOH under section 3810031 Florida Statutes and Florida Administrative Code Chapter 64D-3. Report Status Reported Received Collected P.
If all confinement restrictions are lifted before a vaccine or effective treatments are developed without other measures to suppress new infections the infection rate is expected to rebound rapidly. COVID-19 Pandemic Response Laboratory Data Reporting. The results of certain tests are displayed in a PDF report that provides additional or alternative information about the test results.
Bined manner where a positive result for any antibody the ChemBio DPP COVID-19 IgMIgG Sys-tem is intended to detect was considered as a positive test result and a negative result meant that a sample tested negative for all antibodies the ChemBio DPP COVID-19 IgMIgG System is intended to detect. Till now 67107 cases of coronavirus have been detected globally in 29 countries. Once completed you can sign your fillable form or send for signing.
Biotech Lab and Research Center 5. These PDF reports are not transmitted by HL7 messaging but can be viewed in the MayoACCESS application. The first case of coronavirus disease COVID-19 was first reported from Wuhan China on 31 December 2019.
Persons Mobile Number Sample Id. Interval COVID-19 Virus QUALITATIVE PCR Real Time PCR Negative Negative Interpretation. Use Fill to complete blank online CENTERS FOR DISEASE CONTROL AND PREVENTION GA pdf forms for free.
Manage and care for new cases of COVID-19. Province Region No City Category Functional LabSite 1. As a result of the coronavirus disease COVID-19 pandemic supplies of medical masks and respirators are limited globally.
Medical masks are used in both healthcare and community settings to protect. Countries should prepare to respond to different public health scenarios recognizing that there is no one-size-fits-all approach to managing cases and outbreaks of COVID-19. Reporting of Cycle Threshold Values.
Downloadable Lab Requisition Forms. Captcha This Portal is owned by ICMR the process of authorisation is done on behalf of ICMR Site Designed and Hosted by National Informatics Centre Himachal Pradesh State Centre Shimla. View RATI test report online through covid19cc portal.
Charges for Coronavirus Test in Pakistan. Capital Diagnostic Centre 7. All positive negative and indeterminate COVID-19.
Chughtai Lab Shaheed-e-Millat Road The Radiological and Diagnostic Imaging Centre will also carry out the test by taking a nasopharyngeal swab. To ensure quick lab results we have downloadable requisition forms available. Positive and negative predictive values were.
The main difference in these 2 products is the intended use. A novel coronavirus nCoV is a new strain that has not been previously identified in humans.
Mohammad Hafeez On Twitter After Tested Positive Covid 19 Acc To Pcb Testing Report Yesterday As 2nd Opinion For Satisfaction I Personally Went To Test It Again Along With My Family And Here
Chughtai Lab Chughtai Lab Has Launched Covid 19 Antibody Test For More Information Pls Call Or Whatsapp 03 111 456 789 Facebook

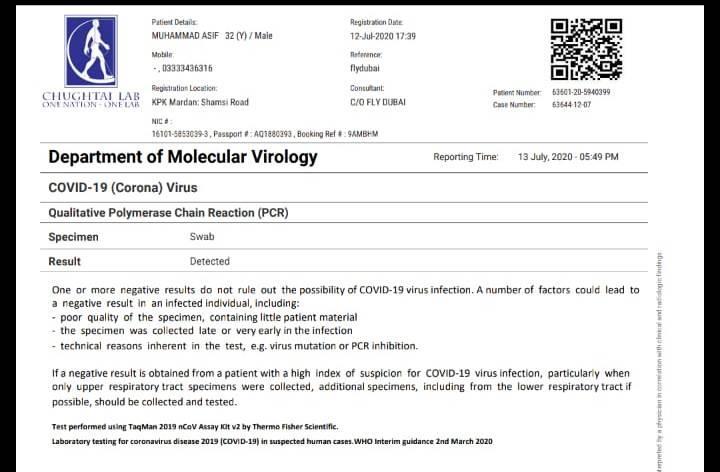
Chughtai Lab Official On Twitter We Have Introduced Encrypted Qr Codes On Our Covid19 Pcr Reports For Report Authentication By Airline Ground Staff The Encrypted Qr Code Can Be Read Offline




Tidak ada komentar:
Posting Komentar